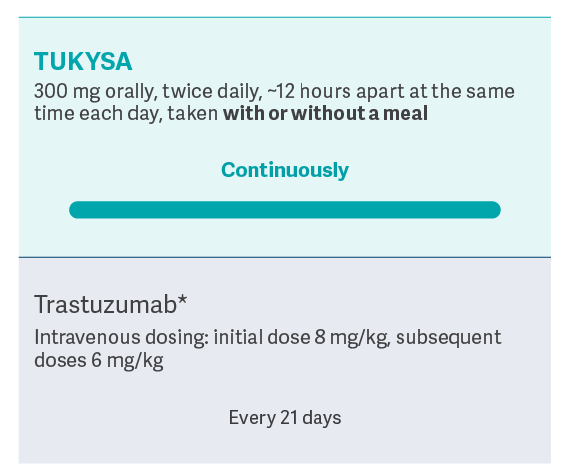
In combination with trastuzumab
TUKYSA is an oral medication taken twice a day, every day, as
part of a chemotherapy-free regimen1,2
- Select patients for treatment of unresectable or metastatic colorectal cancer with TUKYSA based on
the presence of HER2 overexpression or gene amplification, and RAS
WT1 - An FDA-approved test for the detection of HER2 overexpression and gene amplification in patients
with unresectable or metastatic colorectal cancer is not currently
available1
HER2 may also be expressed on healthy cells, which may also be targeted by
anti-HER2 treatments. This may result in adverse reactions, some of which may be
Dosing of TUKYSA® (tucatinib) should continue until disease progression or
unacceptable toxicity1

Monitor ALT, AST, and bilirubin prior to starting TUKYSA, every 3 weeks during treatment, and
as clinically
Additional TUKYSA dosing and administration
information1
- For patients with severe hepatic impairment (Child-Pugh C), the recommended starting dose is 200 mg orally, twice daily
- Avoid concomitant use of strong CYP2C8 inhibitors with TUKYSA. If concomitant use with a strong CYP2C8 inhibitor cannot be avoided, reduce the recommended dosage to 100 mg orally, twice daily. After discontinuation of the strong CYP2C8 inhibitor for 3 elimination half-lives, resume the TUKYSA dose that was taken prior to initiating the inhibitor
- TUKYSA tablets should be swallowed whole; they should not be chewed, crushed, or split prior to swallowing
- If the patient vomits or misses a dose of TUKYSA, the next dose should be taken at the regularly scheduled time
- Please refer to the full Prescribing Information to learn how to modify the dose for select adverse reactions associated with TUKYSA
- For a list of drug-drug interactions, please see Important Safety Information
Consider the TUKYSA regimen in 2L+ RAS WT, HER2+ mCRC
TUKYSA dose modifications for adverse reactions1


Grades based on National Cancer Institute Common Terminology Criteria for Adverse Events Version 4.03.
ULN = upper limit of normal.
Modifying the TUKYSA dose1
Reduce TUKYSA in increments of 50 mg to manage adverse reactions
Recommended starting dose
First dose reduction
Second dose reduction
Third dose reduction
- Some patients may require dose modifications or discontinuations of therapy to manage adverse reactions
- In MOUNTAINEER, 9% of patients had their TUKYSA dose reduced and 6% discontinued TUKYSA due to adverse reactions
- Permanently discontinue TUKYSA in patients unable to tolerate 150 mg orally, twice daily
Trastuzumab dosing may be modified in accordance with its Prescribing Information

Keep current on TUKYSA® (tucatinib). Register for email updates.
Important Safety Information
Warnings and Precautions
- Diarrhea: TUKYSA can cause severe diarrhea including dehydration, hypotension, acute kidney injury, and death. If diarrhea occurs, administer antidiarrheal treatment as clinically indicated. Perform diagnostic tests as clinically indicated to exclude other causes of diarrhea. Based on the severity of the diarrhea, interrupt dose, then dose reduce or permanently discontinue TUKYSA. In MOUNTAINEER, when TUKYSA was given in combination with trastuzumab, diarrhea occurred in 64% of patients, including Grade 3 (3.5%), Grade 2 (10%), and Grade 1 (50%).
- Hepatotoxicity: TUKYSA can cause severe hepatotoxicity. Monitor ALT, AST, and bilirubin prior to starting TUKYSA, every 3 weeks during treatment, and as clinically indicated. Based on the severity of hepatotoxicity, interrupt dose, then dose reduce or permanently discontinue TUKYSA. In MOUNTAINEER, 6% of patients had a bilirubin increase > 3 × ULN (Grade ≥3), 6% had an AST increase > 5 × ULN, and 4.7% had an ALT increase > 5 × ULN. Hepatotoxicity led to dose reduction of TUKYSA in 3.5% of patients and discontinuation of TUKYSA in 2.3% of patients.
- Embryo-Fetal Toxicity: TUKYSA can cause fetal harm. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential, and male patients with female partners of reproductive potential, to use effective contraception during TUKYSA treatment and for 1 week after the last dose.
Adverse Reactions
Serious adverse reactions occurred in 22% of patients; the most common (in ≥2% of patients) were intestinal obstruction (7%), urinary tract infection (3.5%), pneumonia, abdominal pain, and rectal perforation (2.3% each).
Adverse reactions leading to permanent discontinuation of TUKYSA occurred in 6% of patients; the most common (in ≥2% of patients) was increased ALT (2.3%). Adverse reactions leading to dosage interruption occurred in 23% of patients; the most common (in ≥3% of patients) were increased ALT and diarrhea (3.5% each). Adverse reactions leading to dose reduction occurred in 9% of patients; the most common (in ≥2% of patients) were increased ALT and diarrhea (2.3% each).
The most common adverse reactions (≥20%) in patients treated with TUKYSA and trastuzumab were diarrhea, fatigue, rash, nausea, abdominal pain, infusion-related reactions, and pyrexia.
Other adverse reactions (<10%) include epistaxis (7%), weight decreased (7%), oropharyngeal pain (5%), oral dysesthesia (1%), and stomatitis (1%).
Lab Abnormalities
In MOUNTAINEER, Grade ≥3 laboratory abnormalities reported in ≥5% of patients who received TUKYSA were decreased lymphocytes, decreased sodium, increased AST, and increased bilirubin.
The mean increase in serum creatinine was 32% within the first 21 days of treatment with TUKYSA. The serum creatinine increases persisted throughout treatment and were reversible in 87% of patients with values outside normal lab limits upon treatment completion. Consider alternative markers of renal function if persistent elevations in serum creatinine are observed.
Drug Interactions
- Strong CYP3A/Moderate CYP2C8 Inducers: Concomitant use may decrease TUKYSA activity. Avoid concomitant use of TUKYSA.
- Strong or Moderate CYP2C8 Inhibitors: Concomitant use of TUKYSA with a strong CYP2C8 inhibitor may increase the risk of TUKYSA toxicity; avoid concomitant use. Increase monitoring for TUKYSA toxicity with moderate CYP2C8 inhibitors.
- CYP3A Substrates: Concomitant use may increase the toxicity associated with a CYP3A substrate. Avoid concomitant use of TUKYSA with a CYP3A substrate, where minimal concentration changes may lead to serious or life-threatening toxicities. If concomitant use is unavoidable, decrease the CYP3A substrate dosage.
- P-gp Substrates: Concomitant use may increase the toxicity associated with a P-gp substrate. Consider reducing the dosage of P-gp substrates, where minimal concentration changes may lead to serious or life-threatening toxicities.
Use in Specific Populations
- Lactation: Advise women not to breastfeed while taking TUKYSA and for 1 week after the last dose.
- Hepatic Impairment: Reduce the dose of TUKYSA for patients with severe (Child-Pugh C) hepatic impairment.
REF-T1K1163
Indication
TUKYSA is indicated in combination with trastuzumab for the treatment
of adult patients with RAS wild-type, HER2-positive
unresectable or metastatic colorectal cancer that has progressed
following treatment with fluoropyrimidine-, oxaliplatin-, and
irinotecan-based chemotherapy.
This indication is approved under accelerated approval based on tumor
response rate and durability of response. Continued approval for this
indication may be contingent upon verification and description of
clinical benefit in confirmatory trials.
Please see full Prescribing Information.
Important Safety Information
Important Safety Information and Indication
Indication
Warnings and Precautions
Warnings and Precautions
-
Diarrhea:
TUKYSA can cause severe diarrhea including dehydration,
hypotension, acute kidney injury, and death. If diarrhea occurs,
administer antidiarrheal treatment as clinically indicated.
Perform diagnostic tests as clinically indicated to exclude
other causes of diarrhea. Based on the severity of the diarrhea,
interrupt dose, then dose reduce or permanently discontinue
TUKYSA.
In MOUNTAINEER, when TUKYSA was given in combination with trastuzumab, diarrhea occurred in 64% of patients, including Grade 3 (3.5%), Grade 2 (10%), and Grade 1 (50%). - Hepatotoxicity: TUKYSA can cause severe hepatotoxicity. Monitor ALT, AST, and bilirubin prior to starting TUKYSA, every 3 weeks during treatment, and as clinically indicated. Based on the severity of hepatotoxicity, interrupt dose, then dose reduce or permanently discontinue TUKYSA. In MOUNTAINEER, 6% of patients had a bilirubin increase > 3 × ULN (Grade ≥3), 6% had an AST increase > 5 × ULN, and 4.7% had an ALT increase > 5 × ULN. Hepatotoxicity led to dose reduction of TUKYSA in 3.5% of patients and discontinuation of TUKYSA in 2.3% of patients.
-
Embryo-Fetal Toxicity:
TUKYSA can cause fetal harm. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential, and male patients with female partners of reproductive potential, to use effective contraception during TUKYSA treatment and for 1 week after the last dose.
Adverse Reactions
Serious adverse reactions occurred in 22% of patients; the most common (in ≥2% of patients) were intestinal obstruction (7%), urinary tract infection (3.5%), pneumonia, abdominal pain, and rectal perforation (2.3% each).
Adverse reactions leading to permanent discontinuation of TUKYSA occurred in 6% of patients; the most common (in ≥2% of patients) was increased ALT (2.3%). Adverse reactions leading to dosage interruption occurred in 23% of patients; the most common (in ≥3% of patients) were increased ALT and diarrhea (3.5% each). Adverse reactions leading to dose reduction occurred in 9% of patients; the most common (in ≥2% of patients) were increased ALT and diarrhea (2.3% each).
The most common adverse reactions (≥20%) in patients treated with TUKYSA and trastuzumab were diarrhea, fatigue, rash, nausea, abdominal pain, infusion-related reactions, and pyrexia.
Other adverse reactions (<10%) include epistaxis (7%), weight decreased (7%), oropharyngeal pain (5%), oral dysesthesia (1%), and stomatitis (1%).
Lab Abnormalities
In MOUNTAINEER, Grade ≥3 laboratory abnormalities reported in ≥5% of patients who received TUKYSA were decreased lymphocytes, decreased sodium, increased AST, and increased bilirubin.
The mean increase in serum creatinine was 32% within the first 21 days of treatment with TUKYSA. The serum creatinine increases persisted throughout treatment and were reversible in 87% of patients with values outside normal lab limits upon treatment completion. Consider alternative markers of renal function if persistent elevations in serum creatinine are observed.
Drug Interactions
- Strong CYP3A/Moderate CYP2C8 Inducers: Concomitant use may decrease TUKYSA activity. Avoid concomitant use of TUKYSA.
- Strong or Moderate CYP2C8 Inhibitors: Concomitant use of TUKYSA with a strong CYP2C8 inhibitor may increase the risk of TUKYSA toxicity; avoid concomitant use. Increase monitoring for TUKYSA toxicity with moderate CYP2C8 inhibitors.
- CYP3A Substrates: Concomitant use may increase the toxicity associated with a CYP3A substrate. Avoid concomitant use of TUKYSA with a CYP3A substrate, where minimal concentration changes may lead to serious or life-threatening toxicities. If concomitant use is unavoidable, decrease the CYP3A substrate dosage.
- P-gp Substrates: Concomitant use may increase the toxicity associated with a P-gp substrate. Consider reducing the dosage of P-gp substrates, where minimal concentration changes may lead to serious or life-threatening toxicities.
Use in Specific Populations
- Lactation: Advise women not to breastfeed while taking TUKYSA and for 1 week after the last dose.
- Hepatic Impairment: Reduce the dose of TUKYSA for patients with severe (Child-Pugh C) hepatic impairment.
REF-T1K1163
Indication
TUKYSA is indicated in combination with trastuzumab for the
treatment of adult patients with RAS wild-type,
HER2-positive unresectable or metastatic colorectal cancer that
has progressed following treatment with fluoropyrimidine-,
oxaliplatin-, and irinotecan-based chemotherapy.
This indication is approved under accelerated approval based on
tumor response rate and durability of response. Continued approval
for this indication may be contingent upon verification and
description of clinical benefit in confirmatory trials.
Please see full Prescribing Information.
TUKYSA is indicated in combination with trastuzumab for the treatment
of adult patients with RAS wild-type, HER2-positive
unresectable or metastatic colorectal cancer that has progressed
following treatment with fluoropyrimidine-, oxaliplatin-, and
irinotecan-based chemotherapy.
This indication is approved under accelerated approval based on tumor
response rate and durability of response. Continued approval for this
indication may be contingent upon verification and description of
clinical benefit in confirmatory trials.
Please see full Prescribing Information.
1. TUKYSA. Prescribing information. Seagen Inc.; 2023. 2. Trastuzumab. Prescribing information. Genentech, Inc.; 2024. 3. Rubin I, Yarden Y. The basic biology of HER2. Ann Oncol. 2001;12(suppl 1):S3-S8. doi:10.1093/annonc/12.suppl_1.s3 4. Dent SF, Morse A, Burnette S, Guha A, Moore H. Cardiovascular toxicity of novel HER2-targeted therapies in the treatment of breast cancer. Curr Oncol Rep. 2021;23(11):128. doi:10.1007/s11912-021-01114-x
