In combination with trastuzumab and capecitabine
TUKYSA delivered superior results in the primary analysis of
HER2CLIMB1
In patients treated with TUKYSA (tucatinib) + trastuzumab + capecitabine (TUKYSA arm) vs those treated with placebo + trastuzumab + capecitabine (control arm):
Progression-free survival
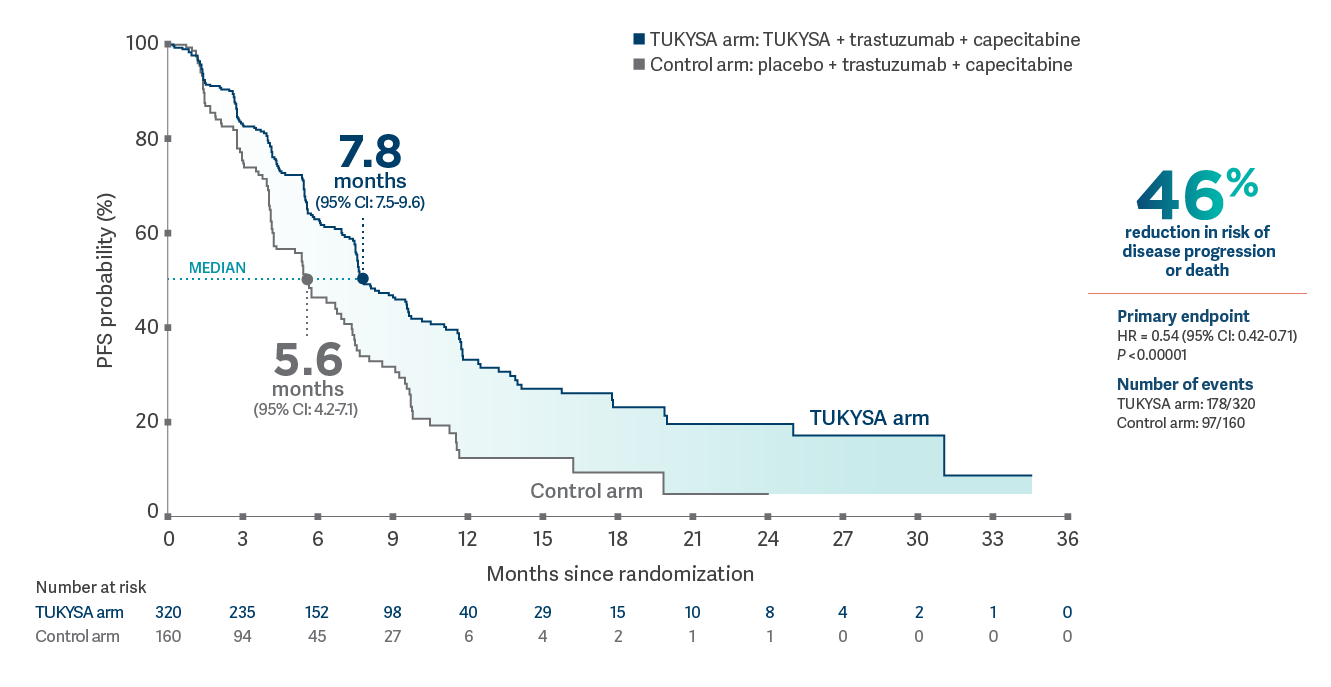
- Primary endpoint: Median PFS: 7.8 months (95% CI: 7.5-9.6) in the TUKYSA arm vs 5.6 months
(95% CI: 4.2-7.1) in the control arm; HR = 0.54 (95% CI: 0.42-0.71); P
<0.000011*
- Secondary endpoint: Median PFS in patients with brain metastases: 7.6 months (95% CI:
6.2-9.5) in the TUKYSA arm vs 5.4 months (95% CI: 4.1-5.7) in the control arm; HR = 0.48 (95% CI: 0.34-0.69);
P
<0.000011
HER2CLIMB: Median overall survival at primary and exploratory follow-up analyses
Results of the prespecified exploratory analysis are descriptive but not conclusive, are not controlled for type 1 error, and should be interpreted with caution. Data cutoff for follow-up analysis was February 8, 2021.2
- These analyses are considered exploratory. No adjustments were made for multiple comparisons in the subgroup analyses
- Small patient numbers can be a limitation of subgroup analyses. These analyses are not intended to demonstrate efficacy in particular subgroups
OS in the total population with updated prespecified data (N = 612)1,2

Efficacy results were consistent across patient subgroups defined by stratification factors and hormone receptor status.1
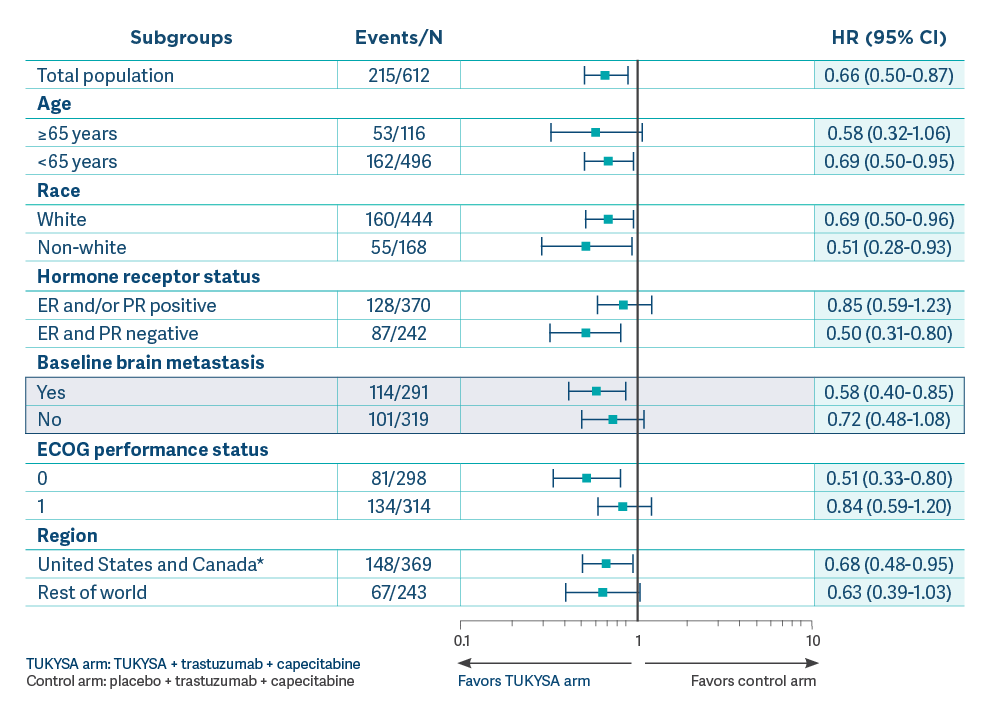
OS results across select subgroups in the primary analysis1,3
- Results of this exploratory subgroup analysis are descriptive but not conclusive, are not controlled for type 1 error, and should be interpreted with caution
- Small patient numbers can be a limitation of subgroup analyses. These analyses are not intended to demonstrate efficacy in particular subgroups
- Data cutoff for primary analysis was September 4, 20193
- These analyses are considered exploratory. No adjustments were made for multiple comparisons in the subgroup analyses
- Small patient numbers can be a limitation of subgroup analyses. These analyses are not intended to demonstrate efficacy in particular subgroups
Median OS in patients with brain metastases (n = 291)4
- 21.6 months (95% CI: 18.1-28.5) in the TUKYSA arm vs 12.5 months (95% CI: 11.2-16.9) in the control arm; HR = 0.60 (95% CI: 0.44-0.81) (exploratory follow-up analysis)
Median OS in patients with visceral metastases (n = 455)5
- 21.6 months (95% CI: 18.1-25.6) in the TUKYSA arm vs 16.9 months (95% CI: 12.3-19.4) in the control arm; HR = 0.70 (95% CI: 0.55-0.89) (exploratory follow-up analysis)
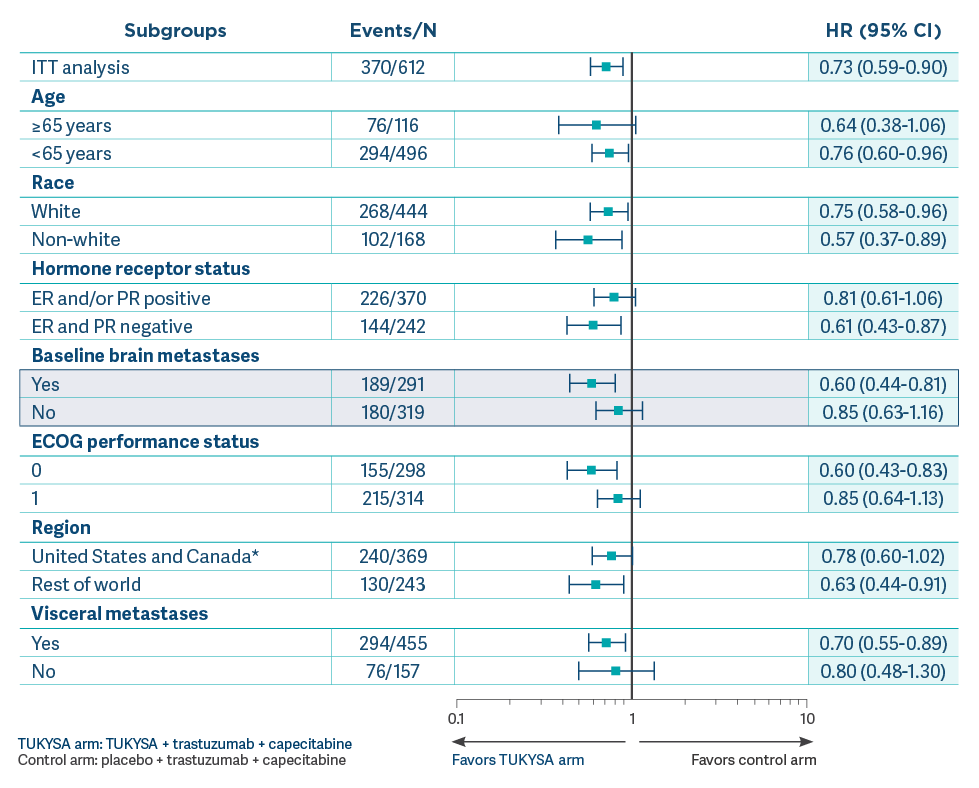
OS results across select subgroups at exploratory follow-up
analysis2,6
- Results of this exploratory subgroup
analysis are descriptive but not conclusive, are not controlled for type 1 error, and should be
interpreted with caution
- Small patient numbers can be a
limitation of subgroup analyses. These analyses are not intended to demonstrate efficacy in
particular subgroups
- Data cutoff for follow-up analysis
was February 8, 20212
OS results across select subgroups at exploratory follow-up analysis2,6
- Results of this exploratory subgroup analysis are descriptive but not conclusive, are not controlled for type 1 error, and should be interpreted with caution
- Small patient numbers can be a limitation of subgroup analyses. These analyses are not intended to demonstrate efficacy in particular subgroups
- Data cutoff for follow-up analysis was February 8, 20212
OS = overall survival.
HER2CLIMB: Exploratory follow-up analyses
HER2CLIMB: Time to new brain lesions or death at exploratory follow-up analysis
- Results of this exploratory analysis are descriptive but not conclusive, and they are not controlled for type 1 error. Due to a high rate of censoring of patients owing to extra-CNS progression, results should be interpreted with caution. Data cutoff for follow-up analysis was February 8, 20214
- Brain lesion development was detected through use of brain MRI scans. All patients received a baseline brain MRI scan. Only patients with a history or baseline presence of brain metastases were scanned at regular intervals throughout the trial. Unless clinically indicated, patients with no history of brain metastasis were not routinely scanned. This depiction is limited to patients who were re-scanned through the trial7
Time from randomization to new brain lesion development as the first site of progression per investigator assessment or death in patients with or without brain metastases at baseline (N = 612)4
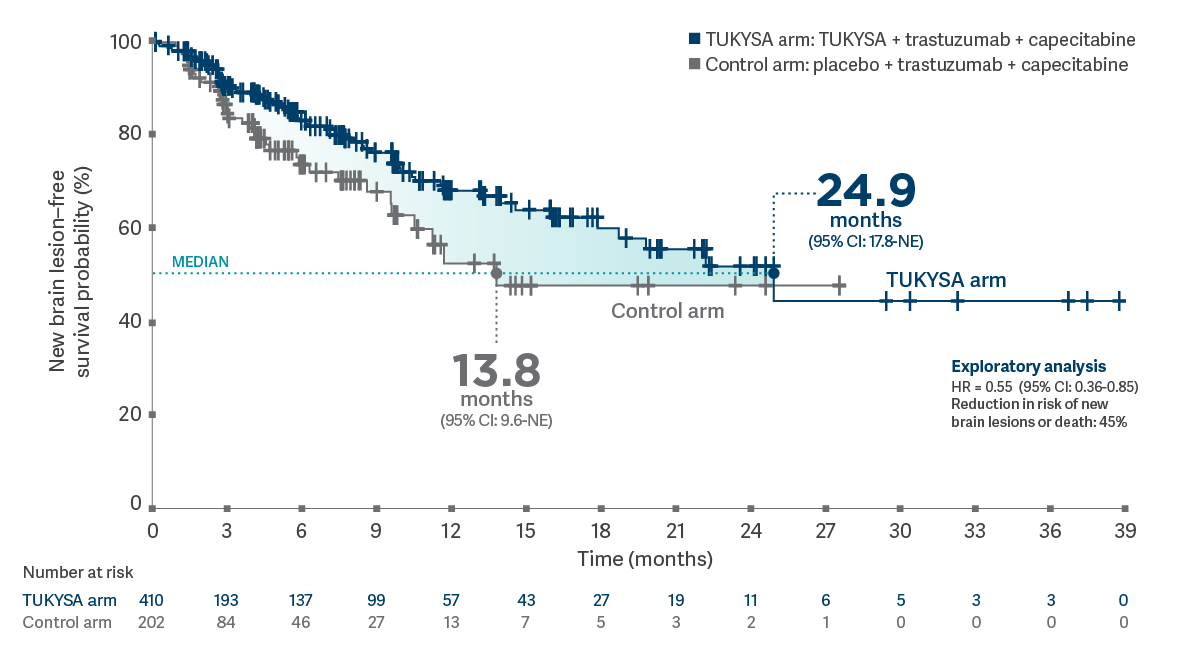
MRI = magnetic resonance imaging; NE = not estimable.
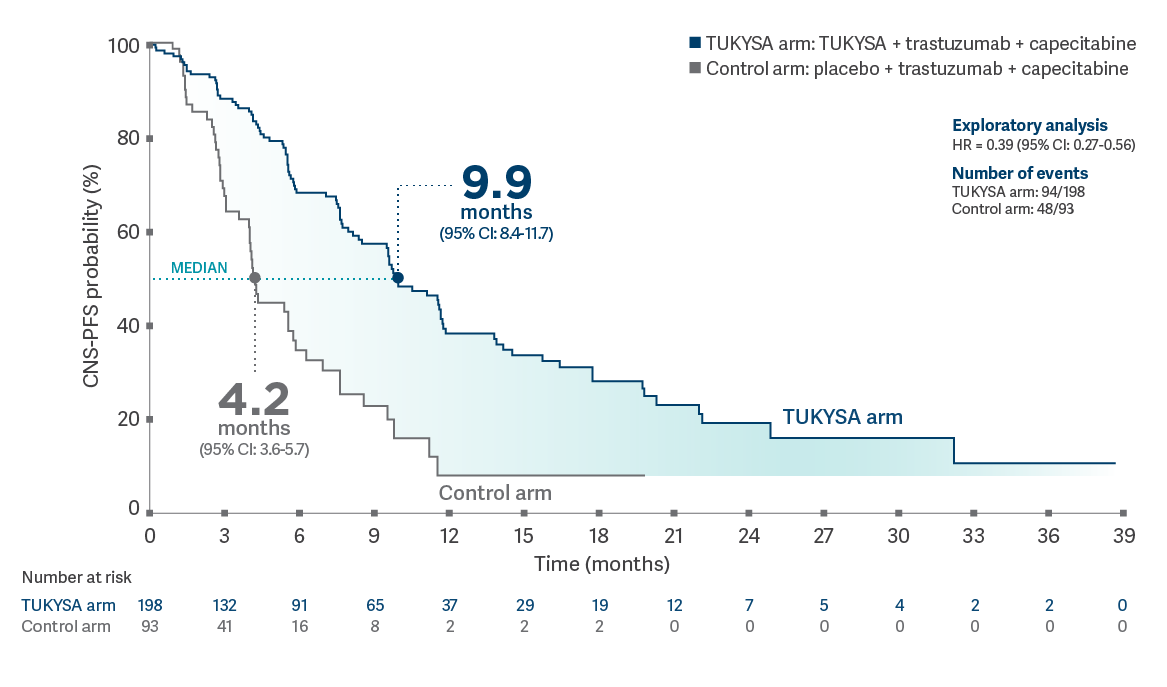
Results of this post-hoc, exploratory
analysis are descriptive, but they are not conclusive, are not controlled for type 1 error, and
should be interpreted with caution. Data cutoff for follow-up analysis was February 8,
2021.4
CNS-PFS in patients with brain metastases at baseline (n = 291)4,8*†
Results of this
post-hoc, exploratory analysis are descriptive, but they are not conclusive, are not controlled for
type 1 error, and should be interpreted with caution. Data cutoff for follow-up analysis was
February 8, 2021.4
ORR-IC in patients with brain metastases at baseline that were measurable for response (n = 75)4,9*
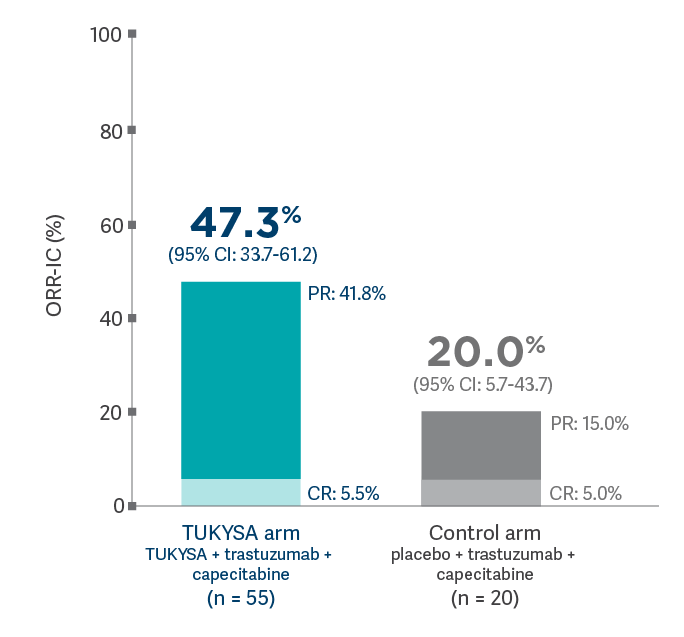
HER2CLIMB: Confirmed tumor response and time to response
- 40.6% (n = 138/340; 95% CI: 35.3-46.0; CR = 0.9%; PR = 39.7%) with TUKYSA + trastuzumab + capecitabine vs 22.8% (n = 39/171; 95% CI: 16.7-29.8; CR = 1.2%; PR = 21.6%) with placebo + trastuzumab + capecitabine; P = 0.00008; (secondary endpoint)
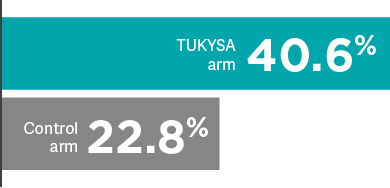
- Median time to response was 6 weeks in both treatment arms
- Results of this exploratory analysis are descriptive only and are not contained in the approved product labeling

HER2CLIMB exploratory analysis: OS in patients with stable
disease as best overall response (n = 343)6*
Results of this exploratory subgroup analysis are descriptive but not conclusive, are not controlled for type 1 error, and should be interpreted with caution.
- Median OS: 21.8 months (95% CI: 18.1-NE) in the TUKYSA arm vs 15.2 months (95% CI: 12.0-20.0) in the
control arm; HR = 0.60 (95% CI:
0.41-0.88)6
- Number of events: 65/217 in the TUKYSA arm vs 53/126 in the control arm
- Stable disease rates in
HER2CLIMB11:
- 45.6% (155/340) in the TUKYSA arm
- 58.5% (100/171) in the control arm
*Analysis included patients in the total population whose confirmed best overall response by BICR was classified
as one of the following: SD or
SD was defined using RECIST, version 1.1, as neither sufficient shrinkage to qualify as a PR (PR was defined as
≥30% decrease) nor sufficient increase to qualify as PD (PD was defined as ≥20%
BICR = blinded independent central review; PD = progressive disease; SD = stable disease.
HER2CLIMB: PFS in patients with and without brain metastases
In combination with trastuzumab and capecitabine
TUKYSA significantly reduced the risk of disease progression or death1
PFS per BICR in the first 480 patients randomized1
In combination with trastuzumab and capecitabine
TUKYSA extended PFS in patients with brain metastases1
PFS per BICR in patients with brain metastases at baseline (n = 291)1,3*
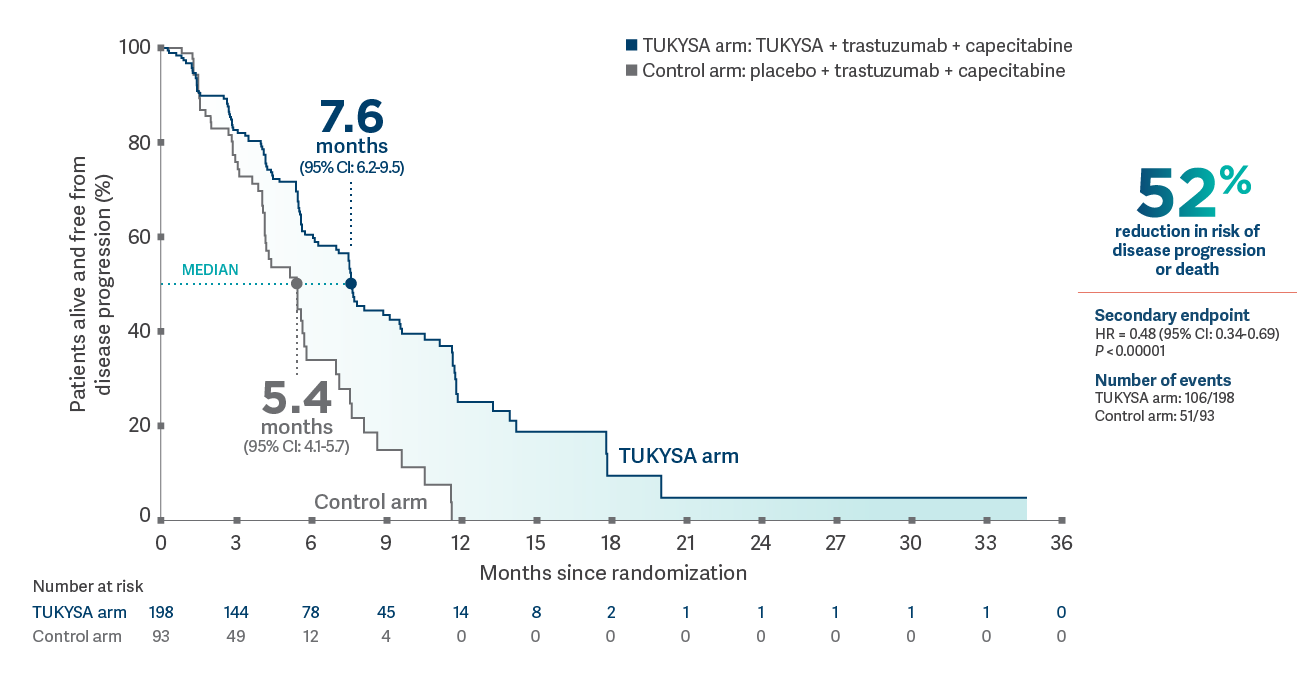
*Analysis includes patients with history or presence of parenchymal brain metastases at baseline, including target and nontarget lesions. Analysis does not include patients with dural lesions only.1
HER2CLIMB exploratory analysis: PFS in patients without brain metastases11
Results of this exploratory subgroup analysis are descriptive but not conclusive, are not controlled for type 1 error, and should be interpreted with caution.PFS per BICR in patients without brain metastases (n = 319)

OS results across select subgroups at exploratory follow-up analysis2,6
- Results of this exploratory subgroup analysis are descriptive but not conclusive, are not controlled for type 1 error, and should be interpreted with caution
- Small patient numbers can be a limitation of subgroup analyses. These analyses are not intended to demonstrate efficacy in particular subgroups
- Data cutoff for follow-up analysis was February 8, 20212

Important Safety Information
Warnings and Precautions
-
Diarrhea: TUKYSA can cause severe diarrhea including
dehydration, hypotension, acute kidney injury, and death. If diarrhea
occurs, administer antidiarrheal treatment as clinically indicated.
Perform diagnostic tests as clinically indicated to exclude other
causes of diarrhea. Based on the severity of the diarrhea, interrupt
dose, then dose reduce or permanently discontinue TUKYSA.
In HER2CLIMB, when TUKYSA was given in combination with trastuzumab and capecitabine, 81% of patients who received TUKYSA experienced diarrhea, including 0.5% with Grade 4 and 12% with Grade 3. Both patients who developed Grade 4 diarrhea subsequently died, with diarrhea as a contributor to death. Median time to onset of the first episode of diarrhea was 12 days and the median time to resolution was 8 days. Diarrhea led to TUKYSA dose reductions in 6% of patients and TUKYSA discontinuation in 1% of patients. Prophylactic use of antidiarrheal treatment was not required on HER2CLIMB. -
Hepatotoxicity: TUKYSA can cause severe
hepatotoxicity. Monitor ALT, AST, and bilirubin prior to starting
TUKYSA, every 3 weeks during treatment, and as clinically indicated.
Based on the severity of hepatotoxicity, interrupt dose, then dose
reduce or permanently discontinue TUKYSA.
In HER2CLIMB, 8% of patients who received TUKYSA had an ALT increase >5 × ULN, 6% had an AST increase >5 × ULN, and 1.5% had a bilirubin increase >3 × ULN (Grade ≥3). Hepatotoxicity led to TUKYSA dose reductions in 8% of patients and TUKYSA discontinuation in 1.5% of patients. - Embryo-Fetal Toxicity: TUKYSA can cause fetal harm. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential, and male patients with female partners of reproductive potential, to use effective contraception during TUKYSA treatment and for 1 week after the last dose.
Adverse Reactions
In HER2CLIMB, serious adverse reactions occurred in 26% of patients
who received TUKYSA; the most common (in ≥2% of patients) were
diarrhea (4%), vomiting (2.5%), nausea (2%), abdominal pain (2%), and
seizure (2%). Fatal adverse reactions occurred in 2% of patients who
received TUKYSA including sudden death, sepsis, dehydration, and
cardiogenic shock.
Adverse reactions led to treatment discontinuation in 6% of patients
who received TUKYSA; the most common (in ≥1% of patients) were
hepatotoxicity (1.5%) and diarrhea (1%). Adverse reactions led to dose
reduction in 21% of patients who received TUKYSA; the most common (in
≥2% of patients) were hepatotoxicity (8%) and diarrhea (6%).
The most common adverse reactions in patients who received TUKYSA
(≥20%) were diarrhea, palmar-plantar erythrodysesthesia, nausea,
hepatotoxicity, vomiting, stomatitis, decreased appetite, anemia, and
rash.
Lab Abnormalities
In HER2CLIMB, Grade ≥3 laboratory abnormalities reported in ≥5% of patients who received TUKYSA were decreased phosphate, increased ALT, decreased potassium, and increased AST.
The mean increase in serum creatinine was 32% within the first 21 days of treatment with TUKYSA. The serum creatinine increases persisted throughout treatment and were reversible upon treatment completion. Consider alternative markers of renal function if persistent elevations in serum creatinine are observed.
Drug Interactions
- Strong CYP3A/Moderate CYP2C8 Inducers: Concomitant use may decrease TUKYSA activity. Avoid concomitant use of TUKYSA.
- Strong or Moderate CYP2C8 Inhibitors: Concomitant use of TUKYSA with a strong CYP2C8 inhibitor may increase the risk of TUKYSA toxicity; avoid concomitant use. Increase monitoring for TUKYSA toxicity with moderate CYP2C8 inhibitors.
- CYP3A Substrates: Concomitant use may increase the toxicity associated with a CYP3A substrate. Avoid concomitant use of TUKYSA where minimal concentration changes may lead to serious or life-threatening toxicities. If concomitant use is unavoidable, decrease the CYP3A substrate dosage.
- P-gp Substrates: Concomitant use may increase the toxicity associated with a P-gp substrate. Consider reducing the dosage of P-gp substrates where minimal concentration changes may lead to serious or life-threatening toxicity.
Use in Specific Populations
- Lactation: Advise women not to breastfeed while taking TUKYSA and for 1 week after the last dose.
- Renal Impairment: Use of TUKYSA in combination with capecitabine and trastuzumab is not recommended in patients with severe renal impairment (CLcr < 30 mL/min), because capecitabine is contraindicated in patients with severe renal impairment.
- Hepatic Impairment: Reduce the dose of TUKYSA for patients with severe (Child-Pugh C) hepatic impairment.
REF-T1K1161
Indication
TUKYSA is indicated in combination with trastuzumab and capecitabine for treatment of adult patients with advanced unresectable or metastatic HER2-positive breast cancer, including patients with brain metastases, who have received one or more prior anti-HER2-based regimens in the metastatic setting.
Please see full Prescribing Information.
Important Safety Information
Important Safety Information + Indication
Indication
Warnings and Precautions
Warnings and Precautions
-
Diarrhea:
TUKYSA can cause severe diarrhea including dehydration,
hypotension, acute kidney injury, and death. If diarrhea occurs,
administer antidiarrheal treatment as clinically indicated.
Perform diagnostic tests as clinically indicated to exclude other
causes of diarrhea. Based on the severity of the diarrhea,
interrupt dose, then dose reduce or permanently discontinue
TUKYSA.
In HER2CLIMB, when TUKYSA was given in combination with trastuzumab and capecitabine, 81% of patients who received TUKYSA experienced diarrhea, including 0.5% with Grade 4 and 12% with Grade 3. Both patients who developed Grade 4 diarrhea subsequently died, with diarrhea as a contributor to death. Median time to onset of the first episode of diarrhea was 12 days and the median time to resolution was 8 days. Diarrhea led to TUKYSA dose reductions in 6% of patients and TUKYSA discontinuation in 1% of patients. Prophylactic use of antidiarrheal treatment was not required on HER2CLIMB. -
Hepatotoxicity:
TUKYSA can cause severe hepatotoxicity. Monitor ALT, AST, and bilirubin prior to starting TUKYSA, every 3 weeks during treatment, and as clinically indicated. Based on the severity of hepatotoxicity, interrupt dose, then dose reduce or permanently discontinue TUKYSA.
In HER2CLIMB, 8% of patients who received TUKYSA had an ALT increase >5 × ULN, 6% had an AST increase >5 × ULN, and 1.5% had a bilirubin increase >3 × ULN (Grade ≥3). Hepatotoxicity led to TUKYSA dose reductions in 8% of patients and TUKYSA discontinuation in 1.5% of patients.
-
Embryo-Fetal Toxicity:
TUKYSA can cause fetal harm. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential, and male patients with female partners of reproductive potential, to use effective contraception during TUKYSA treatment and for 1 week after the last dose.
Adverse Reactions
In HER2CLIMB, serious adverse reactions occurred in 26% of
patients who received TUKYSA; the most common (in ≥2% of
patients) were diarrhea (4%), vomiting (2.5%), nausea (2%),
abdominal pain (2%), and seizure (2%). Fatal adverse reactions
occurred in 2% of patients who received TUKYSA including sudden
death, sepsis, dehydration, and cardiogenic shock.
Adverse reactions led to treatment discontinuation in 6% of
patients who received TUKYSA; the most common (in ≥1% of
patients) were hepatotoxicity (1.5%) and diarrhea (1%). Adverse
reactions led to dose reduction in 21% of patients who received
TUKYSA; the most common (in ≥2% of patients) were hepatotoxicity
(8%) and diarrhea (6%).
The most common adverse reactions in patients who received
TUKYSA (≥20%) were diarrhea, palmar-plantar erythrodysesthesia,
nausea, hepatotoxicity, vomiting, stomatitis, decreased
appetite, anemia, and rash.
Lab Abnormalities
In HER2CLIMB, Grade ≥3 laboratory abnormalities reported in ≥5% of patients who received TUKYSA were decreased phosphate, increased ALT, decreased potassium, and increased AST.
The mean increase in serum creatinine was 32% within the first 21 days of treatment with TUKYSA. The serum creatinine increases persisted throughout treatment and were reversible upon treatment completion. Consider alternative markers of renal function if persistent elevations in serum creatinine are observed.
Drug Interactions
- Strong CYP3A/Moderate CYP2C8 Inducers: Concomitant use may decrease TUKYSA activity. Avoid concomitant use of TUKYSA.
- Strong or Moderate CYP2C8 Inhibitors: Concomitant use of TUKYSA with a strong CYP2C8 inhibitor may increase the risk of TUKYSA toxicity; avoid concomitant use. Increase monitoring for TUKYSA toxicity with moderate CYP2C8 inhibitors.
- CYP3A Substrates: Concomitant use may increase the toxicity associated with a CYP3A substrate. Avoid concomitant use of TUKYSA where minimal concentration changes may lead to serious or life-threatening toxicities. If concomitant use is unavoidable, decrease the CYP3A substrate dosage.
- P-gp Substrates: Concomitant use may increase the toxicity associated with a P-gp substrate. Consider reducing the dosage of P-gp substrates where minimal concentration changes may lead to serious or life-threatening toxicity.
Use in Specific Populations
- Lactation: Advise women not to breastfeed while taking TUKYSA and for 1 week after the last dose.
- Renal Impairment: Use of TUKYSA in combination with capecitabine and trastuzumab is not recommended in patients with severe renal impairment (CLcr < 30 mL/min), because capecitabine is contraindicated in patients with severe renal impairment.
- Hepatic Impairment: Reduce the dose of TUKYSA for patients with severe (Child-Pugh C) hepatic impairment.
REF-T1K1161
Indication
TUKYSA is indicated in combination with trastuzumab and capecitabine for treatment of adult patients with advanced unresectable or metastatic HER2-positive breast cancer, including patients with brain metastases, who have received one or more prior anti-HER2-based regimens in the metastatic setting.
Please see full Prescribing Information.
TUKYSA is indicated in combination with trastuzumab and capecitabine for treatment of adult patients with advanced unresectable or metastatic HER2-positive breast cancer, including patients with brain metastases, who have received one or more prior anti-HER2-based regimens in the metastatic setting.
Please see full Prescribing Information.
1. TUKYSA. Prescribing information. Seagen Inc.; 2023. 2. Curigliano G, Mueller V, Borges V, et al. Tucatinib versus placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB): final overall survival analysis. Ann Oncol. 2022;33(3):321-329. doi:10.1016/j.annonc.2021.12.005 3. Murthy RK, Loi S, Okines A, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382(7):597-609. doi:10.1056/NEJMoa1914609 4. Lin NU, Murthy RK, Abramson V, et al. Tucatinib vs placebo, both in combination with trastuzumab and capecitabine, for previously treated ERBB2 (HER2)-positive metastatic breast cancer in patients with brain metastases: updated exploratory analysis of the HER2CLIMB randomized clinical trial. JAMA Oncol. 2023;9(2):197-205. doi:10.1001/jamaoncol.2022.5610 5. Curigliano G, Mueller V, Borges V, et al. Updated results of tucatinib vs placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB). Poster presented at: American Society of Clinical Oncology Annual Meeting; June 4-8, 2021. 6. Data on file. Seagen Inc. 7. Murthy RK, Loi S, Okines A, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382(7):597-609. Protocol. doi:10.1056/NEJMoa1914609 8. Lin NU, Murthy RK, Abramson V, et al. Updated results of tucatinib vs placebo added to trastuzumab and capecitabine for patients with previously treated HER2+ metastatic breast cancer with brain metastases (HER2CLIMB). Poster presented at: San Antonio Breast Cancer Symposium; December 7-10, 2021; San Antonio, TX. 9. Lin NU, Borges V, Anders C, et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol. 2020;38(23):2610-2619. doi:10.1200/JCO.20.00775 10. Curigliano G, Murthy R, Loi S, et al. Tucatinib vs placebo added to trastuzumab and capecitabine in previously treated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB). Ann Oncol. 2020;31(suppl 2):S62-S63. doi:10.1016/j.annonc.2020.03.238 11. Murthy RK, Loi S, Okines A, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382(7):597-609. Supplementary appendix. doi:10.1056/NEJMoa1914609 12. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi:10.1016/j.ejca.2008.10.026

