HER2CLIMB: A pivotal trial representative of a heterogeneous population1,2
✓ Global ✓ Randomized ✓ Double-Blind ✓ Controlled
✓ Pivotal Trial
Patients with unresectable locally advanced or metastatic HER2+ breast cancer who had received prior trastuzumab, pertuzumab, and T-DM1 separately or in combination, in the neoadjuvant, adjuvant, or metastatic setting, were eligible to
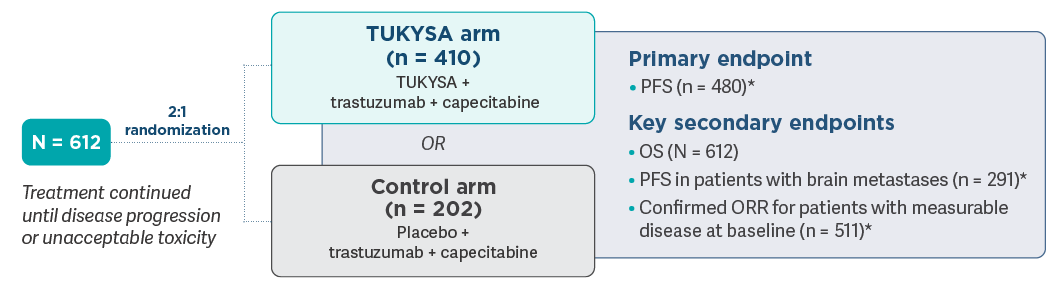

- Dosing was repeated every 21 days: TUKYSA (tucatinib), 300 mg orally, twice daily, or placebo, twice daily; trastuzumab, 6 mg/kg intravenously, once every 21 days with an initial dose of 8 mg/kg (subcutaneous dosing was also allowed); capecitabine, 1000 mg/m2 orally, twice daily, on Days 1-141
- Primary endpoint was PFS evaluated in accordance with RECIST, version 1.1, by means of BICR in the
first 480 patients enrolled. Secondary endpoints were assessed in the full study
population1 - Patients were stratified according to presence or history of brain metastases (yes or no), ECOG
performance status score (0 or 1), and geographic region
(United States, Canada, or rest of
world)1
*Evaluated in accordance with RECIST, version 1.1, by means of
BICR = blinded independent central review; ECOG = Eastern Cooperative Oncology Group; HER = human
epidermal growth factor receptor; ORR = objective response rate; OS = overall survival; PFS =
progression-free survival; RECIST = Response Evaluation Criteria in Solid Tumors; T-DM1 =
ado-trastuzumab emtansine.
HER2CLIMB follow-up analysis3
- HER2CLIMB follow-up analysis included a prespecified exploratory analysis to evaluate OS, PFS (by
investigator assessment), and safety in the total study population (N = 612) at ~2 years from the
last patient randomized
- 12.9% of patients in the placebo arm (26/202) crossed over to receive TUKYSA in combination with
trastuzumab and capecitabine, with the first patient crossover in February 2020
- The median overall study follow-up was 29.6 months (data cutoff: February 8, 2021)
- Because formal testing of all alpha-controlled endpoints was considered final at the primary analysis, data from this prespecified updated analysis are for descriptive purposes only
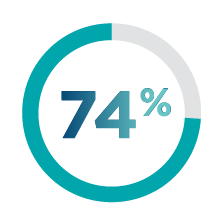
HER2CLIMB was the first randomized trial of a systemic therapy to study patients with HER2+ MBC
and active brain metastases1,4-9
metastases at
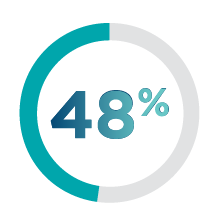
Of those
patients who had brain metastases at
- 40% had stable brain metastases
- 60% had active brain metastases
- 23% had untreated progressing brain metastases
- 37% had treated but progressing brain metastases
metastases at
HER2CLIMB assessed a broad population of patients with HER2+ MBC2,10
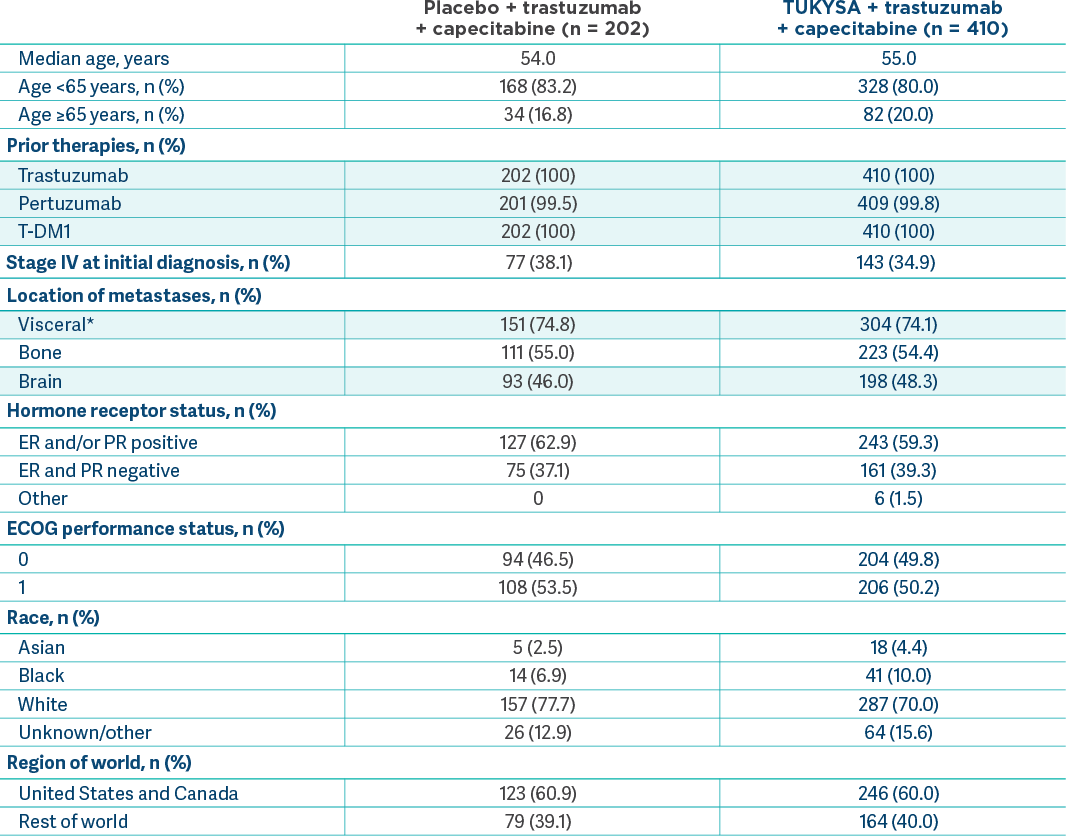
In HER2CLIMB, patients in the TUKYSA arm received a median of 3 prior lines of therapy in the metastatic setting (range: 1-14)2†
Important Safety Information
Warnings and Precautions
-
Diarrhea: TUKYSA can cause severe diarrhea including
dehydration, hypotension, acute kidney injury, and death. If diarrhea
occurs, administer antidiarrheal treatment as clinically indicated.
Perform diagnostic tests as clinically indicated to exclude other
causes of diarrhea. Based on the severity of the diarrhea, interrupt
dose, then dose reduce or permanently discontinue TUKYSA.
In HER2CLIMB, when TUKYSA was given in combination with trastuzumab and capecitabine, 81% of patients who received TUKYSA experienced diarrhea, including 0.5% with Grade 4 and 12% with Grade 3. Both patients who developed Grade 4 diarrhea subsequently died, with diarrhea as a contributor to death. Median time to onset of the first episode of diarrhea was 12 days and the median time to resolution was 8 days. Diarrhea led to TUKYSA dose reductions in 6% of patients and TUKYSA discontinuation in 1% of patients. Prophylactic use of antidiarrheal treatment was not required on HER2CLIMB. -
Hepatotoxicity: TUKYSA can cause severe
hepatotoxicity. Monitor ALT, AST, and bilirubin prior to starting
TUKYSA, every 3 weeks during treatment, and as clinically indicated.
Based on the severity of hepatotoxicity, interrupt dose, then dose
reduce or permanently discontinue TUKYSA.
In HER2CLIMB, 8% of patients who received TUKYSA had an ALT increase >5 × ULN, 6% had an AST increase >5 × ULN, and 1.5% had a bilirubin increase >3 × ULN (Grade ≥3). Hepatotoxicity led to TUKYSA dose reductions in 8% of patients and TUKYSA discontinuation in 1.5% of patients. - Embryo-Fetal Toxicity: TUKYSA can cause fetal harm. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential, and male patients with female partners of reproductive potential, to use effective contraception during TUKYSA treatment and for 1 week after the last dose.
Adverse Reactions
In HER2CLIMB, serious adverse reactions occurred in 26% of patients
who received TUKYSA; the most common (in ≥2% of patients) were
diarrhea (4%), vomiting (2.5%), nausea (2%), abdominal pain (2%), and
seizure (2%). Fatal adverse reactions occurred in 2% of patients who
received TUKYSA including sudden death, sepsis, dehydration, and
cardiogenic shock.
Adverse reactions led to treatment discontinuation in 6% of patients
who received TUKYSA; the most common (in ≥1% of patients) were
hepatotoxicity (1.5%) and diarrhea (1%). Adverse reactions led to dose
reduction in 21% of patients who received TUKYSA; the most common (in
≥2% of patients) were hepatotoxicity (8%) and diarrhea (6%).
The most common adverse reactions in patients who received TUKYSA
(≥20%) were diarrhea, palmar-plantar erythrodysesthesia, nausea,
hepatotoxicity, vomiting, stomatitis, decreased appetite, anemia, and
rash.
Lab Abnormalities
In HER2CLIMB, Grade ≥3 laboratory abnormalities reported in ≥5% of patients who received TUKYSA were decreased phosphate, increased ALT, decreased potassium, and increased AST.
The mean increase in serum creatinine was 32% within the first 21 days of treatment with TUKYSA. The serum creatinine increases persisted throughout treatment and were reversible upon treatment completion. Consider alternative markers of renal function if persistent elevations in serum creatinine are observed.
Drug Interactions
- Strong CYP3A/Moderate CYP2C8 Inducers: Concomitant use may decrease TUKYSA activity. Avoid concomitant use of TUKYSA.
- Strong or Moderate CYP2C8 Inhibitors: Concomitant use of TUKYSA with a strong CYP2C8 inhibitor may increase the risk of TUKYSA toxicity; avoid concomitant use. Increase monitoring for TUKYSA toxicity with moderate CYP2C8 inhibitors.
- CYP3A Substrates: Concomitant use may increase the toxicity associated with a CYP3A substrate. Avoid concomitant use of TUKYSA where minimal concentration changes may lead to serious or life-threatening toxicities. If concomitant use is unavoidable, decrease the CYP3A substrate dosage.
- P-gp Substrates: Concomitant use may increase the toxicity associated with a P-gp substrate. Consider reducing the dosage of P-gp substrates where minimal concentration changes may lead to serious or life-threatening toxicity.
Use in Specific Populations
- Lactation: Advise women not to breastfeed while taking TUKYSA and for 1 week after the last dose.
- Renal Impairment: Use of TUKYSA in combination with capecitabine and trastuzumab is not recommended in patients with severe renal impairment (CLcr < 30 mL/min), because capecitabine is contraindicated in patients with severe renal impairment.
- Hepatic Impairment: Reduce the dose of TUKYSA for patients with severe (Child-Pugh C) hepatic impairment.
REF-T1K1161
Indication
TUKYSA is indicated in combination with trastuzumab and capecitabine for treatment of adult patients with advanced unresectable or metastatic HER2-positive breast cancer, including patients with brain metastases, who have received one or more prior anti-HER2-based regimens in the metastatic setting.
Please see full Prescribing Information.
Important Safety Information
Important Safety Information + Indication
Indication
Warnings and Precautions
Warnings and Precautions
-
Diarrhea:
TUKYSA can cause severe diarrhea including dehydration,
hypotension, acute kidney injury, and death. If diarrhea occurs,
administer antidiarrheal treatment as clinically indicated.
Perform diagnostic tests as clinically indicated to exclude other
causes of diarrhea. Based on the severity of the diarrhea,
interrupt dose, then dose reduce or permanently discontinue
TUKYSA.
In HER2CLIMB, when TUKYSA was given in combination with trastuzumab and capecitabine, 81% of patients who received TUKYSA experienced diarrhea, including 0.5% with Grade 4 and 12% with Grade 3. Both patients who developed Grade 4 diarrhea subsequently died, with diarrhea as a contributor to death. Median time to onset of the first episode of diarrhea was 12 days and the median time to resolution was 8 days. Diarrhea led to TUKYSA dose reductions in 6% of patients and TUKYSA discontinuation in 1% of patients. Prophylactic use of antidiarrheal treatment was not required on HER2CLIMB. -
Hepatotoxicity:
TUKYSA can cause severe hepatotoxicity. Monitor ALT, AST, and bilirubin prior to starting TUKYSA, every 3 weeks during treatment, and as clinically indicated. Based on the severity of hepatotoxicity, interrupt dose, then dose reduce or permanently discontinue TUKYSA.
In HER2CLIMB, 8% of patients who received TUKYSA had an ALT increase >5 × ULN, 6% had an AST increase >5 × ULN, and 1.5% had a bilirubin increase >3 × ULN (Grade ≥3). Hepatotoxicity led to TUKYSA dose reductions in 8% of patients and TUKYSA discontinuation in 1.5% of patients.
-
Embryo-Fetal Toxicity:
TUKYSA can cause fetal harm. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential, and male patients with female partners of reproductive potential, to use effective contraception during TUKYSA treatment and for 1 week after the last dose.
Adverse Reactions
In HER2CLIMB, serious adverse reactions occurred in 26% of
patients who received TUKYSA; the most common (in ≥2% of
patients) were diarrhea (4%), vomiting (2.5%), nausea (2%),
abdominal pain (2%), and seizure (2%). Fatal adverse reactions
occurred in 2% of patients who received TUKYSA including sudden
death, sepsis, dehydration, and cardiogenic shock.
Adverse reactions led to treatment discontinuation in 6% of
patients who received TUKYSA; the most common (in ≥1% of
patients) were hepatotoxicity (1.5%) and diarrhea (1%). Adverse
reactions led to dose reduction in 21% of patients who received
TUKYSA; the most common (in ≥2% of patients) were hepatotoxicity
(8%) and diarrhea (6%).
The most common adverse reactions in patients who received
TUKYSA (≥20%) were diarrhea, palmar-plantar erythrodysesthesia,
nausea, hepatotoxicity, vomiting, stomatitis, decreased
appetite, anemia, and rash.
Lab Abnormalities
In HER2CLIMB, Grade ≥3 laboratory abnormalities reported in ≥5% of patients who received TUKYSA were decreased phosphate, increased ALT, decreased potassium, and increased AST.
The mean increase in serum creatinine was 32% within the first 21 days of treatment with TUKYSA. The serum creatinine increases persisted throughout treatment and were reversible upon treatment completion. Consider alternative markers of renal function if persistent elevations in serum creatinine are observed.
Drug Interactions
- Strong CYP3A/Moderate CYP2C8 Inducers: Concomitant use may decrease TUKYSA activity. Avoid concomitant use of TUKYSA.
- Strong or Moderate CYP2C8 Inhibitors: Concomitant use of TUKYSA with a strong CYP2C8 inhibitor may increase the risk of TUKYSA toxicity; avoid concomitant use. Increase monitoring for TUKYSA toxicity with moderate CYP2C8 inhibitors.
- CYP3A Substrates: Concomitant use may increase the toxicity associated with a CYP3A substrate. Avoid concomitant use of TUKYSA where minimal concentration changes may lead to serious or life-threatening toxicities. If concomitant use is unavoidable, decrease the CYP3A substrate dosage.
- P-gp Substrates: Concomitant use may increase the toxicity associated with a P-gp substrate. Consider reducing the dosage of P-gp substrates where minimal concentration changes may lead to serious or life-threatening toxicity.
Use in Specific Populations
- Lactation: Advise women not to breastfeed while taking TUKYSA and for 1 week after the last dose.
- Renal Impairment: Use of TUKYSA in combination with capecitabine and trastuzumab is not recommended in patients with severe renal impairment (CLcr < 30 mL/min), because capecitabine is contraindicated in patients with severe renal impairment.
- Hepatic Impairment: Reduce the dose of TUKYSA for patients with severe (Child-Pugh C) hepatic impairment.
REF-T1K1161
Indication
TUKYSA is indicated in combination with trastuzumab and capecitabine for treatment of adult patients with advanced unresectable or metastatic HER2-positive breast cancer, including patients with brain metastases, who have received one or more prior anti-HER2-based regimens in the metastatic setting.
Please see full Prescribing Information.
TUKYSA is indicated in combination with trastuzumab and capecitabine for treatment of adult patients with advanced unresectable or metastatic HER2-positive breast cancer, including patients with brain metastases, who have received one or more prior anti-HER2-based regimens in the metastatic setting.
Please see full Prescribing Information.
References
1. TUKYSA. Prescribing information. Seagen Inc.; 2023.
2. Murthy RK, Loi S, Okines A, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382(7):597-609. doi:10.1056/NEJMoa1914609 3. Curigliano G, Mueller V, Borges V, et al. Tucatinib versus placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB): final overall survival analysis. Ann Oncol. 2022;33(3):321-329. doi:10.1016/j.annonc.2021.12.005 4. Neratinib. Prescribing information. Puma Biotechnology, Inc.; 2022.
5. Fam-trastuzumab deruxtecan-nxki. Prescribing information. Daiichi Sankyo, Inc.; 2025.
6. Swain SM, Baselga J, Kim S-B, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724-734. doi:10.1056/NEJMoa1413513 7. Rugo HS, Im S-A, Cardoso F, et al. Efficacy of margetuximab vs trastuzumab in patients with pretreated ERBB2-positive advanced breast cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2021;7(4):573-584. doi:10.1001/jamaoncol.2020.7932
8. Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733-2743. doi:10.1056/NEJMoa064320
9. Krop IE, Lin NU, Blackwell K, et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol. 2015;26(1):113-119. doi:10.1093/annonc/mdu486
10. Data on file. Seagen Inc.

